1. Introduction
Electrocatalytic CO2 reduction reaction (CO2RR) to generate high-value-added multi-carbon products (C2+) is an effective way to achieve the resourceful utilization of CO2. Copper-based catalysts have been extensively studied for their unique catalytic activity. However, due to the complexity of electron transfer, the slow kinetics of *CO intermediate dimerization, and the competitive hydrogen evolution and C1 pathways, the Faradaic efficiency of C2+ products still fails to meet practical demands under high current densities. Introducing a second metal into the Cu matrix to modulate the electronic structure can effectively enhance CO2 reduction to C2+ products. Ag has been proven to significantly regulate and improve the catalytic performance of copper-based catalysts through tandem catalytic strategies or surface alloying strategies. However, Ag is usually used as the second ideal metal site to accelerate the conversion of CO2 to CO and to increase the coverage of the *CO intermediate required for C2+ formation, while neglecting the role of adding Ag atoms in forming asymmetric Cu sites. Therefore, the key influence of Ag components on the intrinsic activity of Cu-based bimetallic CO2RR alloys is still unclear, which seriously hinders the understanding of the CO2RR mechanism and the rational design of catalysts.
2. Introduction to Achievements
The single-atom silver-doped copper nanosheet catalysts (CuAgx% NSs) were prepared in this paper to enhance the performance of CO2 reduction to C2+ products. Combining theoretical calculations with in situ spectroscopy results, we found that the introduction of single Ag atom induces asymmetric Cu sites, which can maintain a low *CO adsorption energy barrier while significantly reducing the C-C coupling energy barrier, thereby enhancing the coverage of *CO and *OCCO intermediates. The results show that CuAg0.123% NSs exhibit high C2+ Faradaic efficiencies (FE) of 77.5% and 71.3% under conditions of 300 and 500 mA cm−2, respectively. This work emphasizes the importance of asymmetric Cu sites in regulating *CO adsorption based on the Ag-doping effect and provides a new pathway for designing highly efficient C2+-selective catalytic sites.
3. Graphic and textual guidance
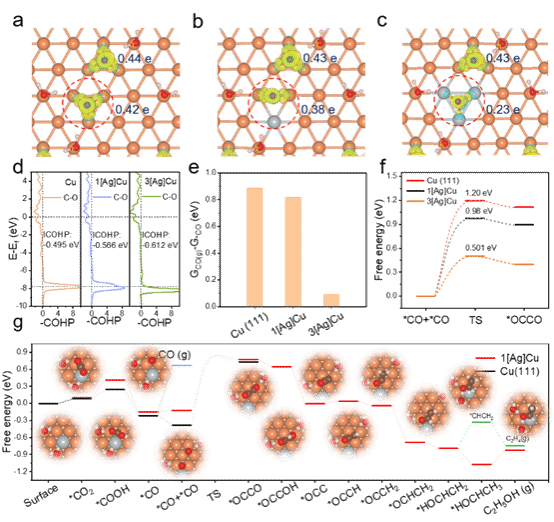
Figure 1. DFT Theoretical Calculations
Theoretical calculations reveal that compared to the Cu (111) and 3[Ag]Cu surfaces, the 1[Ag]Cu surface can better stabilize the *CO while significantly reducing the C-C coupling energy barrier, thereby promoting the formation of C2+ products.
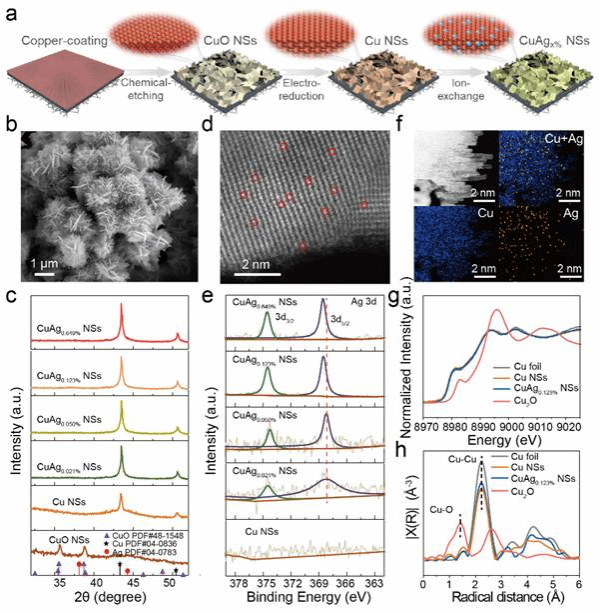
Figure 2. Preparation and Characterization of CuAg0.123% NSs Catalysts
The study prepared single-atom Ag-doped Cu nanosheet catalysts via an ion-exchange method. Characterization data from aberration-corrected electron microscopy and synchrotron radiation confirmed the successful doping of single-atom Ag on the surface of the Cu nanosheet catalysts, as well as the regulation of different Ag contents.
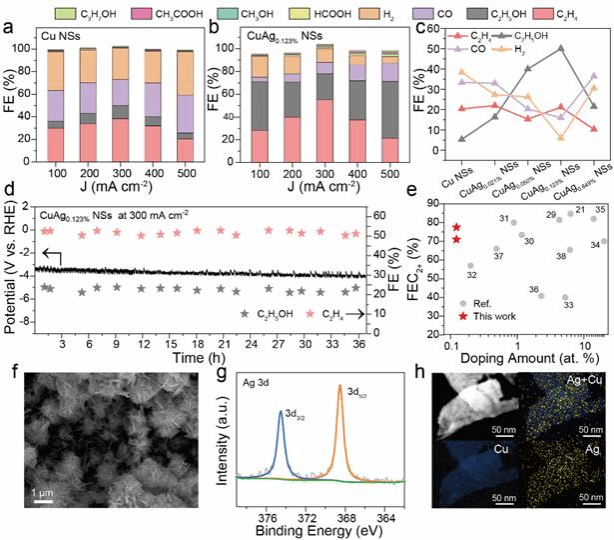
Figure 3. CO2RR Performance Testing of CuAgx% NSs Catalysts
The CO2RR performance of CuAgx% NSs catalysts was tested. The results show that the CuAg0.123% NSs catalyst exhibited a higher yield of multi-carbon products under higher current density conditions and demonstrated better stability. Compared with the doping content of similar materials reported in the literature, its performance has significant advantages.
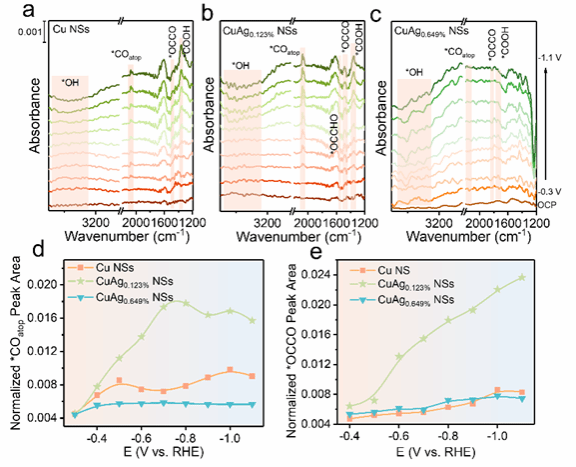
Figure 4. In situ Spectroscopy Characterization and Analysis
To further investigate the catalytic mechanism, this paper tested the in situ infrared spectra. The results show that the CuAg0.123% NSs catalyst surface has higher peak areas of *CO and *OCCO, demonstrating that the doping of single-atom Ag enhances the surface coverage of *CO and *OCCO, thereby promoting the formation of multicarbon products. The conclusion is consistent with the results of theoretical calculations.
Finally, the authors provided a summary and outlook. This paper demonstrates the promotion of electrocatalytic CO2 reduction to multicarbon products by modulating asymmetric copper sites. Density Functional Theory (DFT) calculations indicate that single-atom Ag doping can induce the formation of asymmetric Cu sites, reduce the adsorption energy of *CO, and further facilitate the coupling process of *CO + *CO. In situ infrared results confirm the crucial role of asymmetric Cu sites in CuAg0.123% NSs in promoting the formation of *CO intermediates and their subsequent dimerization, thereby facilitating the generation of C2+ products. The results show that CuAg0.123% NSs achieve a high Faradaic efficiency for C2+ (FEC2+) of 71.3% at 500 mA cm−2. More importantly, at an industrial-level current density of 300 mA cm−2, FEC2+ reaches 77.5%, with long-term stability demonstrated. Therefore, this work provides new insights into how atomically dispersed Ag regulates asymmetric Cu sites to enhance CO2RR performance.