1、Introduction:
Lithium sulfur (Li-S) batteries have the advantages of high theoretical energy density and low cost, and are the next generation electrochemical energy storage devices with great potential for application, which have received widespread attention from researchers. However, the intermediate products of charge and discharge, lithium polysulfides (LiPSs), are highly soluble in organic electrolytes and cause a "shuttle effect" under concentration gradients, resulting in low utilization of sulfur active materials, negative electrode corrosion, and short cycle life, seriously hindering the commercialization process of Li-S batteries. Research has shown that constructing highly active catalysts to accelerate the conversion of LiPSs into charge discharge products and avoid their accumulation in the electrolyte is an effective strategy for suppressing shuttle effects. This work utilizes the method of flash Joule heating to prepare heterostructure catalysts such as W-W2C/G. Due to the different work functions of W (5.08 eV) and W2C (6.31 eV), a spontaneous internal electric field is generated at the heterojunction interface, accelerating the movement of electrons and ions and promoting the sulfur reduction reaction (SRR) process, effectively suppressing the shuttle effect.
2、Introduction to Achievements:
This study synthesized W-W2C heterostructures (W-W2C/G) as catalytic intermediate layers on graphene substrates using a one-step ultrafast flash Joule heating method. Published a research paper titled "Flash Joule Heating: A Promising Method for Preparing Heterogeneous Catalysts to Inhibit Polysulfide Shutting in Li-S Batteries" in the internationally renowned journal Advanced Science. This paper focuses on the advantages of preparing heterostructure catalysts using ultrafast Joule heating. The internal electric field generated at the heterostructure interface accelerates the conversion of LiPSs and effectively suppresses the shuttle effect. This study provides new possibilities for designing effective catalysts to improve the conversion rate of polysulfides.
3、Graphic and textual guidance:
Key point one: Preparation of heterogeneous catalysts by flash Joule heating
Heterogeneous catalysts such as W-W2C/G and Mo-Mo2C were synthesized using the flash Joule heating method, and this method is universal and can achieve rapid in-situ synthesis of large-scale heterogeneous catalysts. The ultrafast heating/cooling process and shorter growth time promote the uniform dispersion of heterogeneous catalyst nanoparticles, forming a large number of heterogeneous interfaces, effectively preventing the degradation of catalytic activity caused by aggregation.
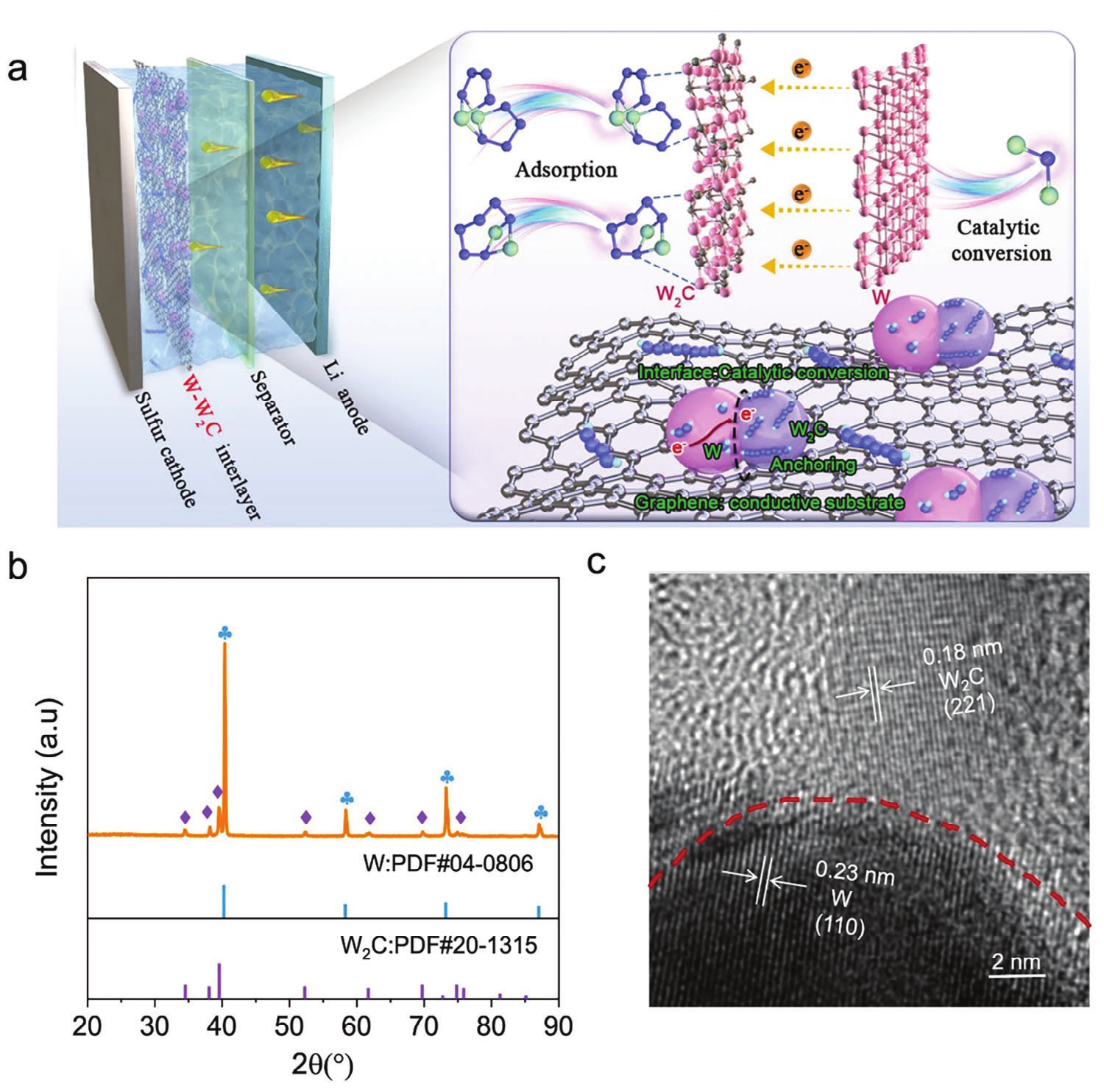
Figure 1. Schematic diagram and material characterization of W-W2C/G heterostructure accelerating LiPSs conversion
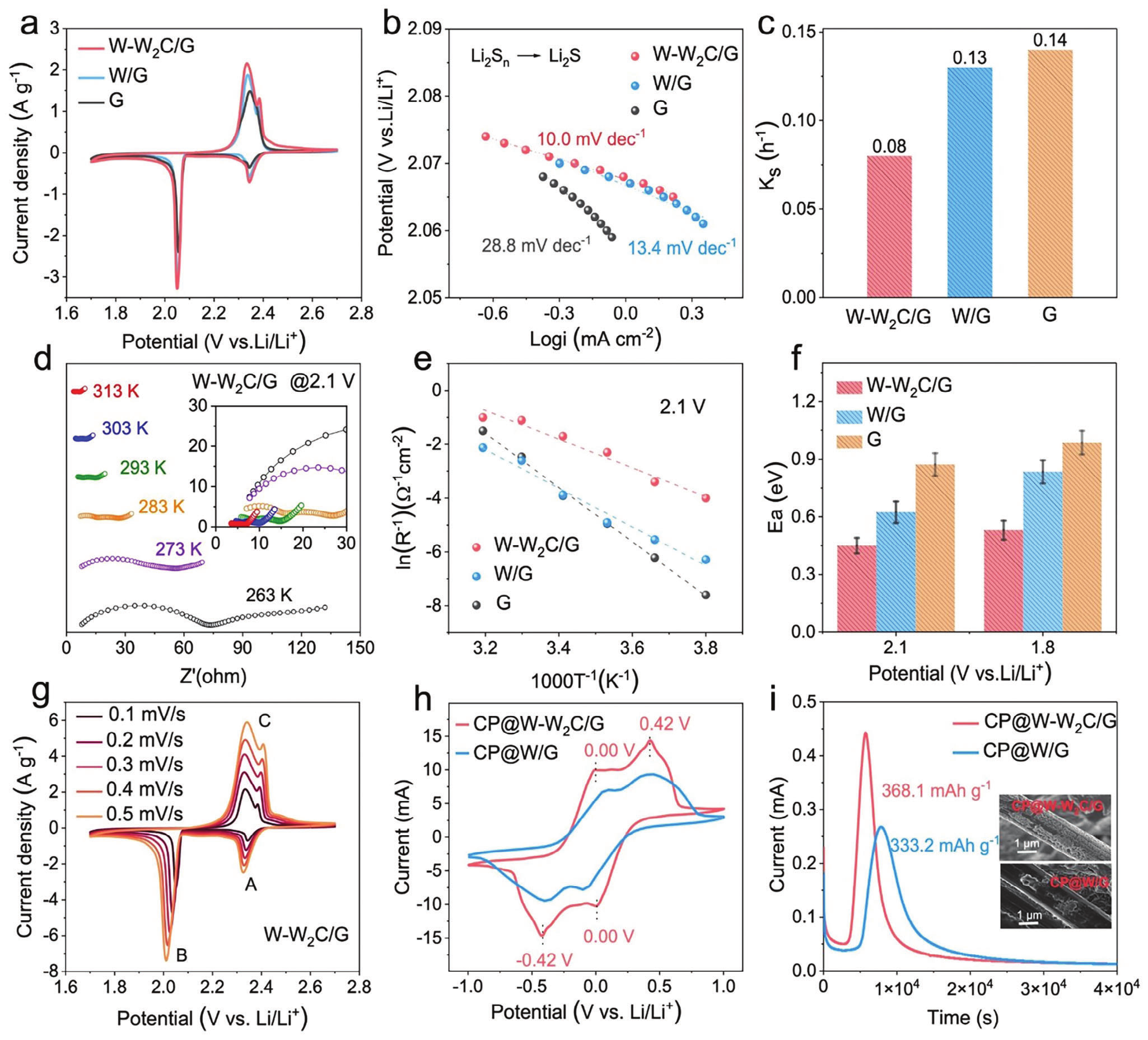
Figure 2. Electrocatalytic effect
Key point two: Excellent catalytic activity of W-W2C/G heterostructure
Density functional theory (DFT) calculations show that the work function of W (5.08 eV) is smaller than that of W2C (6.31 eV), driving electrons to flow from W to W2C, thereby generating a spontaneous internal electric field at the interface, which can accelerate electron transfer and ion diffusion. This structure optimizes the interface electronic states of W-W2C heterostructures, enhances the adsorption and conversion processes of LiPSs, and effectively suppresses the shuttle of LiPSs. The electrochemical experimental results showed that the W-W2C heterostructure catalyst reduced the activation energy of sulfur reduction reaction and the overpotential of charge and discharge; The shuttle coefficient of polysulfides decreased from 0.14 to 0.08 h-1, greatly improving the utilization efficiency of sulfur active substances.
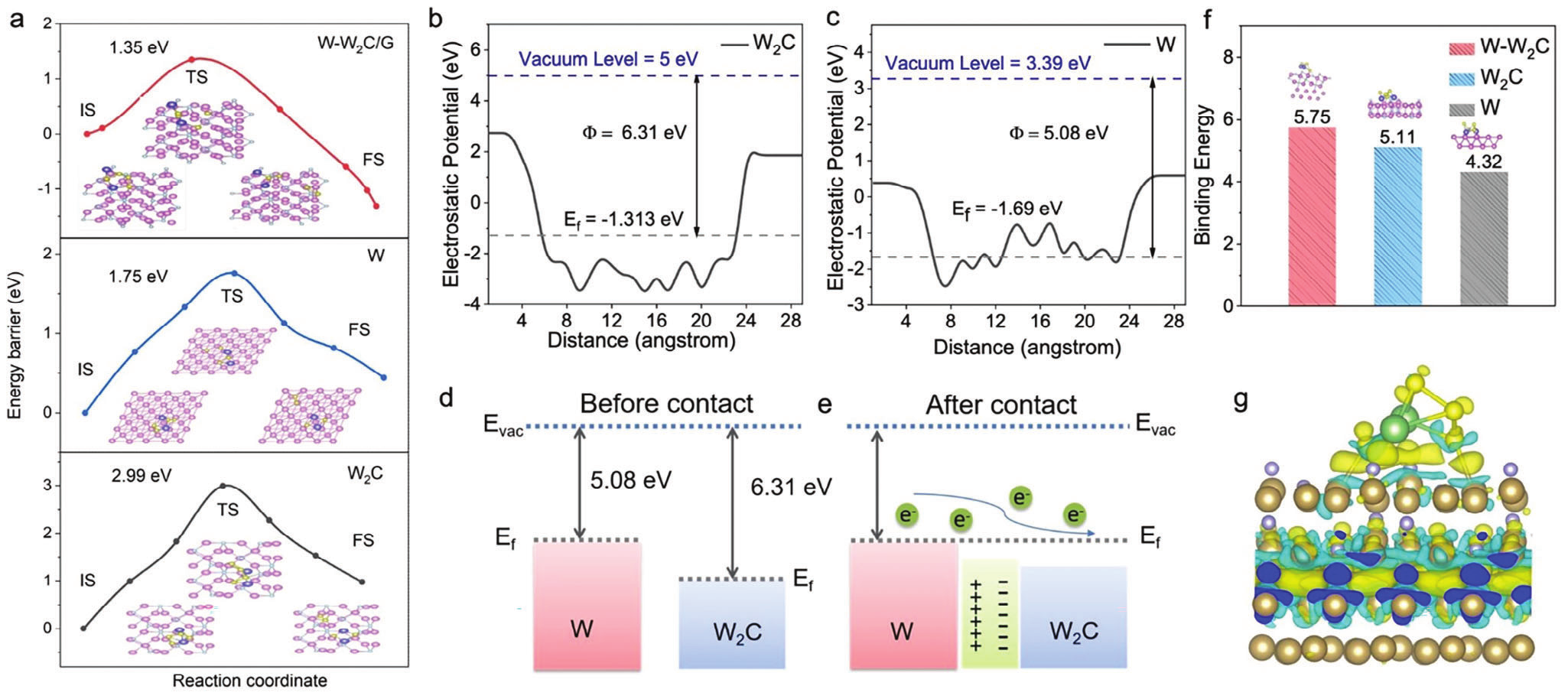
Figure 3. Theoretical Calculation
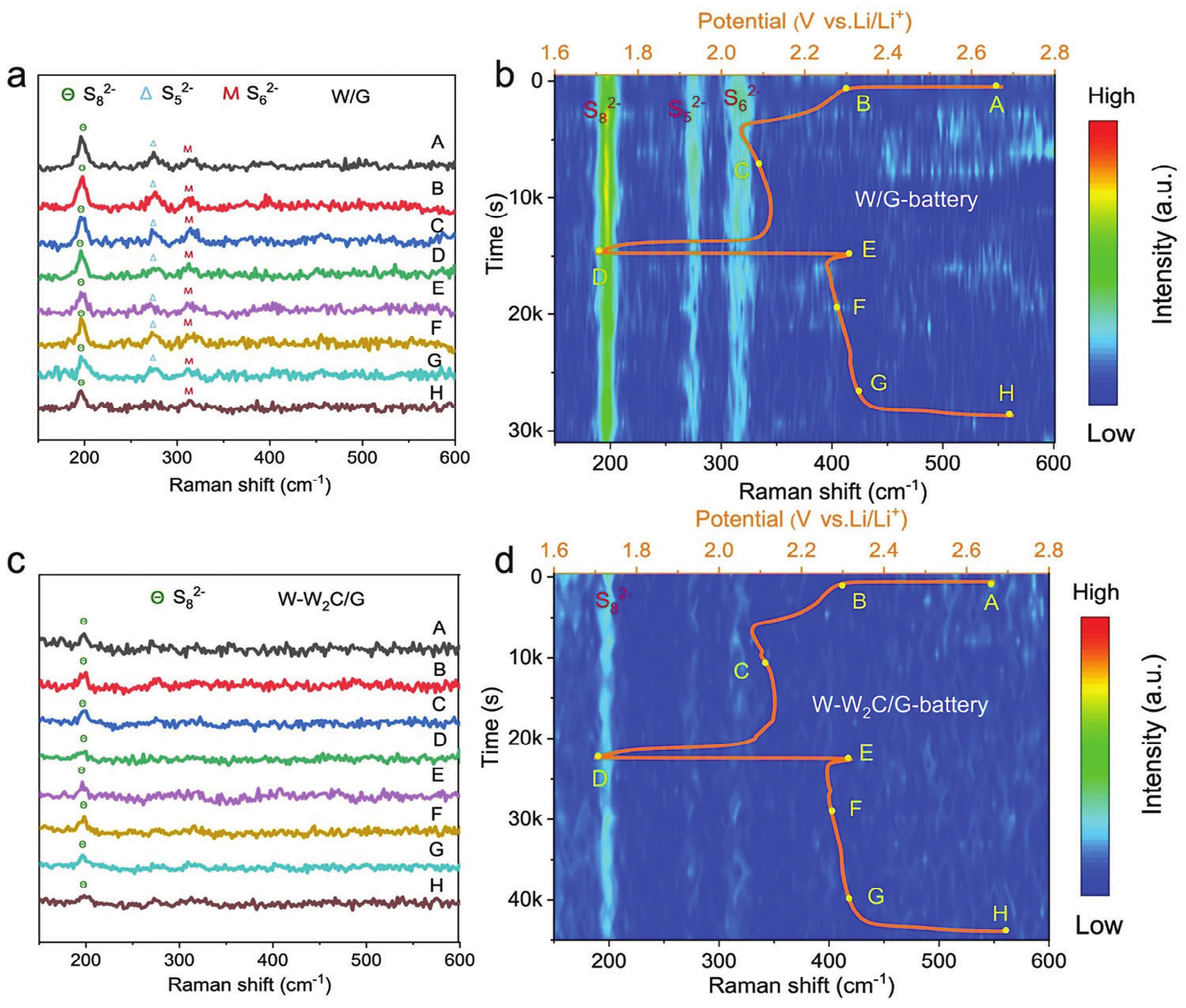
Figure 4. In situ Raman spectroscopy demonstrates inhibition of polysulfide shuttle
Key point three: Excellent electrochemical performance
Thanks to the efficient catalytic activity of the W-W2C heterostructure, Li-S batteries exhibit excellent rate performance (665 mAh g-1, 5.0 C) and can stably cycle for over 1000 cycles at a high rate of 3.0 C, with an average capacity decay rate of only 0.06% per cycle. In addition, the battery can achieve a first cycle surface capacity of up to 10.9 mAh cm-2 (1381.4 mAh g-1) at high sulfur loading (7.9 mg ccm-2) and low electrolyte usage (9.0 μL mg-1). After stable cycling at 0.2 C for 100 cycles, the capacity retention rate is still 80.6%.
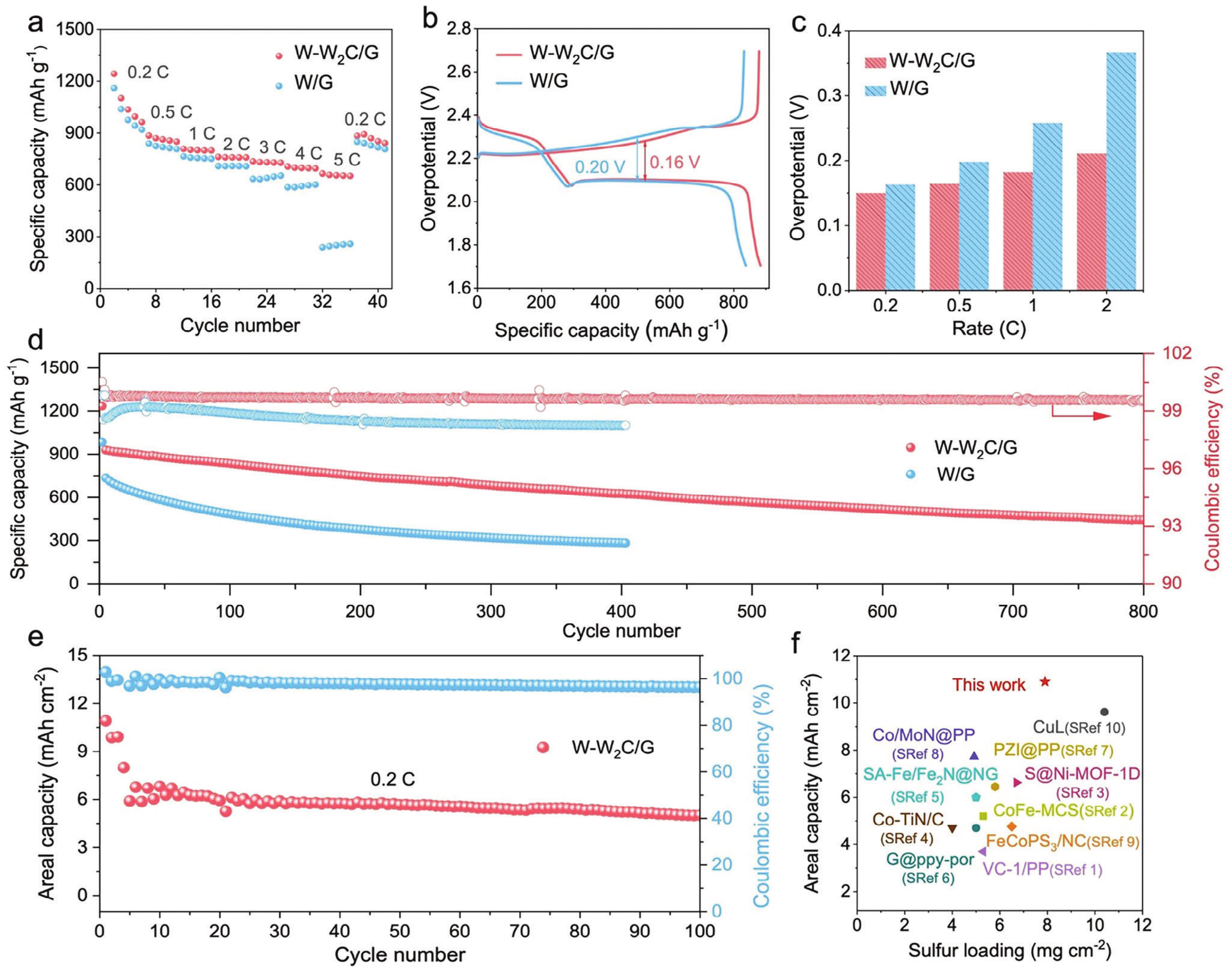
Figure 5. Electrochemical performance
Finally, the author provided a summary and outlook. This study introduces a general method for synthesizing heterostructure catalysts using ultrafast flash Joule heating technology and verifies their effectiveness as intermediate layers for Li-S battery catalysis. A series of experimental measurements, including in-situ Raman performance and theoretical calculations, have verified that the W-W2C heterostructure has a high adsorption capacity for LiPSs and generates an internal electric field at the heterostructure interface, ensuring rapid charge transfer, greatly suppressing the shuttle effect of LiPSs, enhancing SRR kinetics, and improving electrochemical performance. This work provides a new strategy for designing practical electrocatalysts for high-performance lithium sulfur batteries.